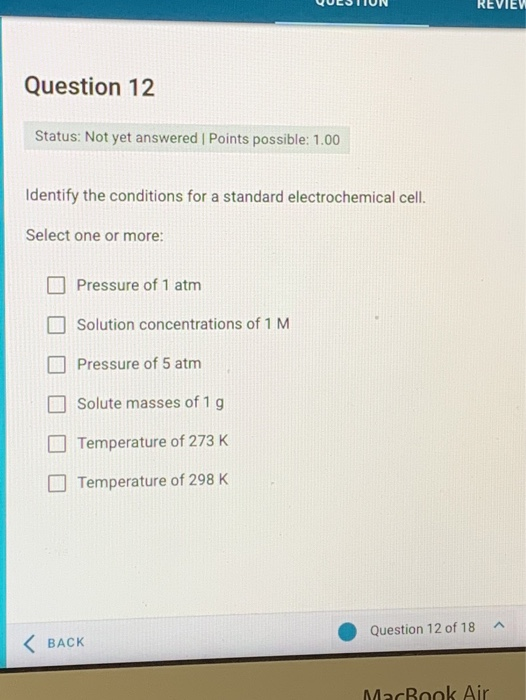
Identify the Conditions for a Standard Electrochemical Cell.
Pressure of 1 atm Solution concentrations of 1 M Pressure of 5 atm Solute masses of 1 g. The energy of a system that is available to do work at a constant temperature and pressure.
Electrochemical Cell Definition Overview Expii
A standard cell potential E is the voltage of an electrochemical cell in which aU species are in their standard states.
. Ad Browse Discover Thousands of Science Book Titles for Less. Find the standard cell potential for an electrochemical cell with the. Conditions for a standard electrochemical cell are - Pressure of 1 atm Solution concentrations of 1 M Temperature of 298 K Explanation- An el View the full answer.
Solution concentrations of 1 M Pressure of 1 atm Pressure of 5 atm Solute masses of 1 g Temperature. An example of an electrochemical cell is a standard cell of 15-volt which is used to give power to many electrical appliances such as digital cameras clocks AC remotes etc. G Identify the conditions for a standard electrochemical cell.
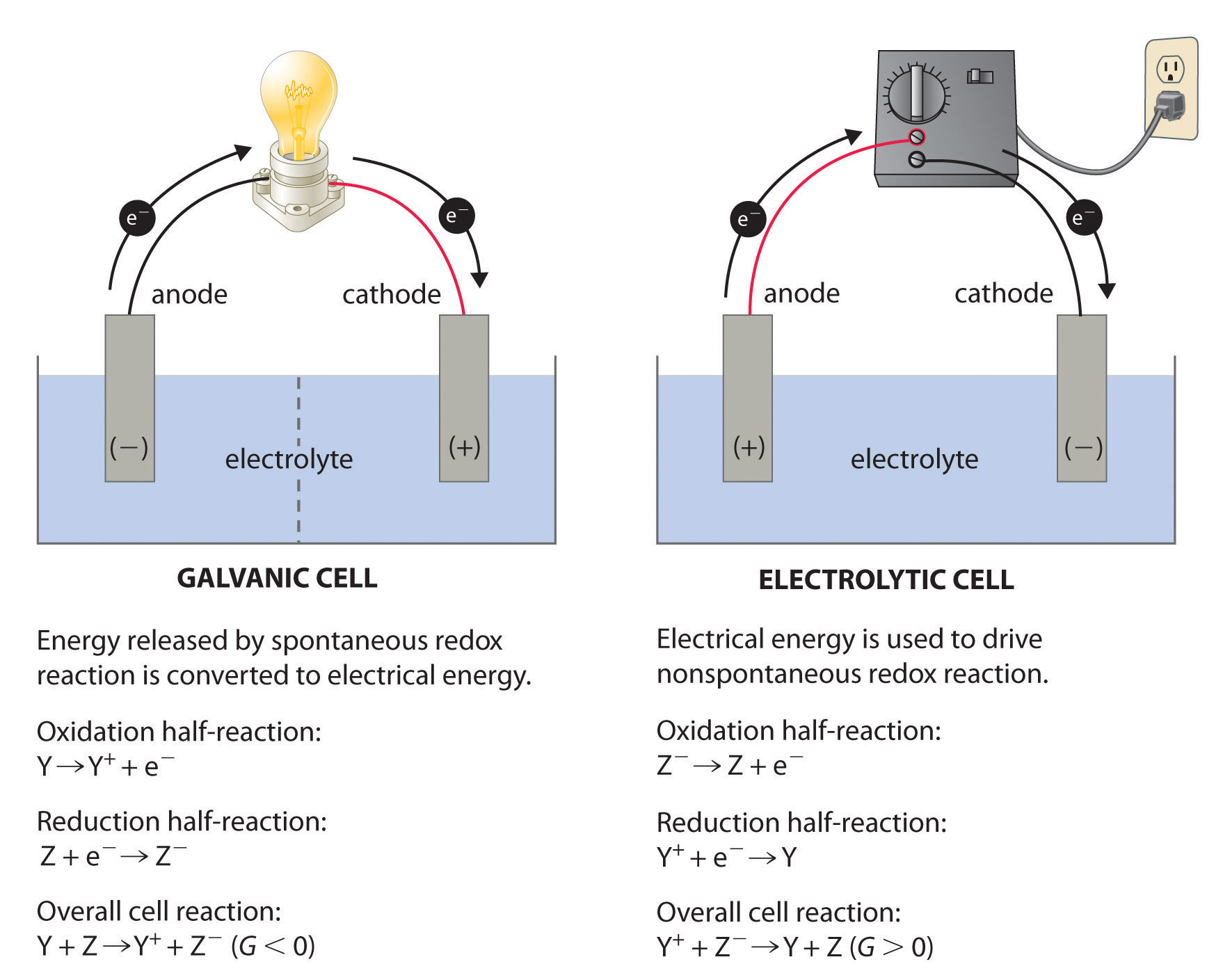
Eocell Eoreduction Eooxidation. Electrochemical cells can take place under standard conditions or non-standard conditions in both electrons always flow from the anode to the cathode. Identify the conditions for a standard electrochemical cell.
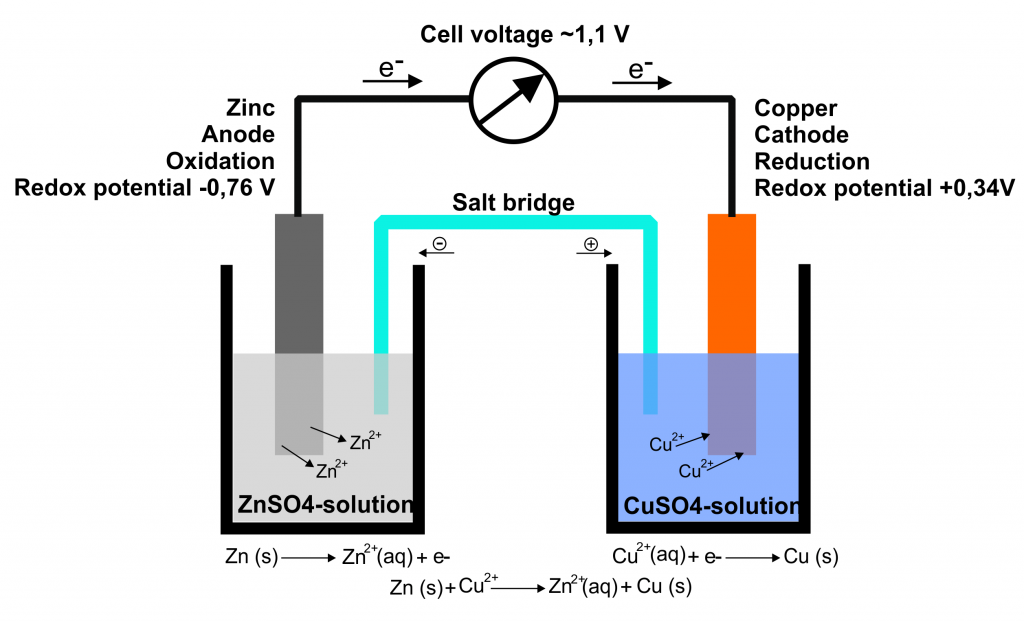
Identify the conditions for a standard electrochemical cell. Identify the conditions for a standard. In the pictured cell the side containing zinc is the _____ and the side containing copper is the _____.
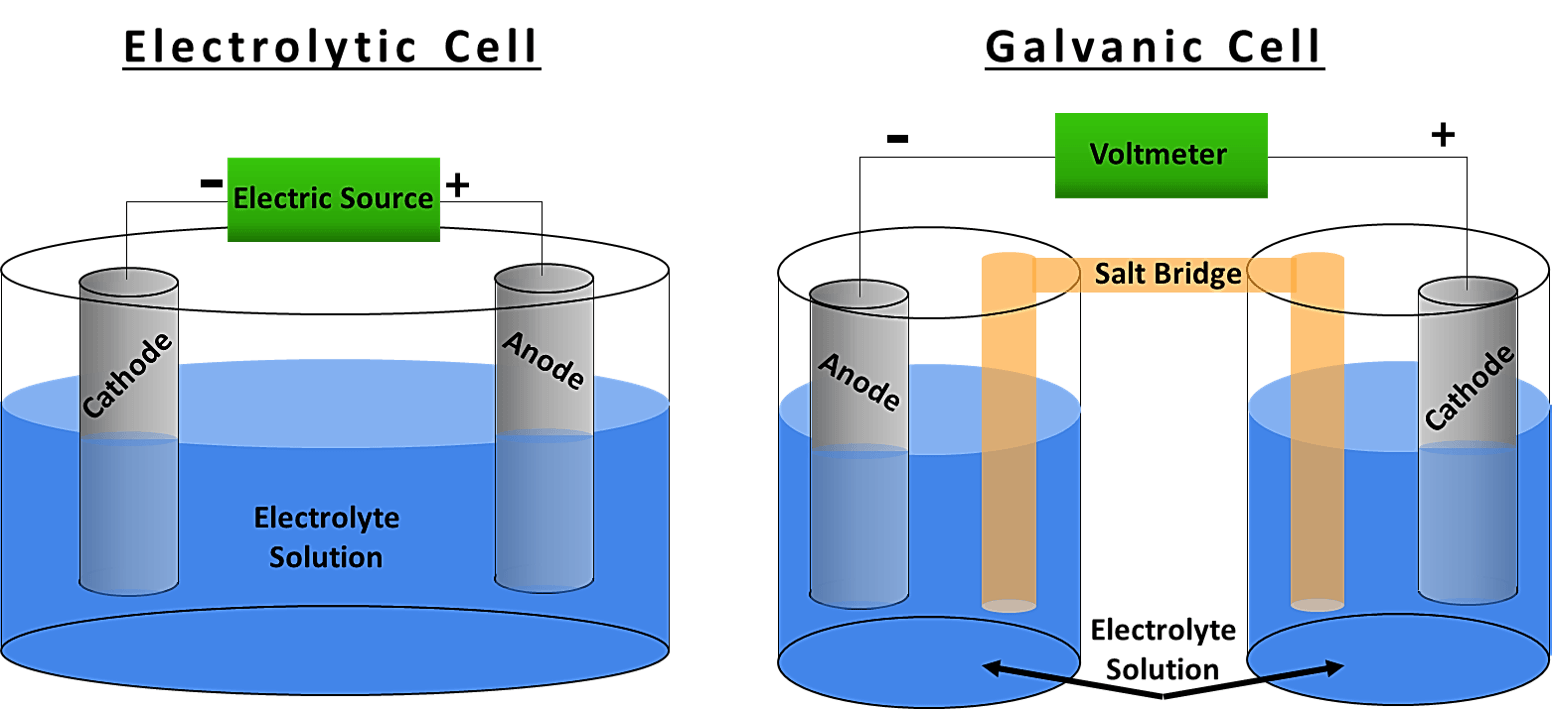
To do this chemists use the standard cell potential E cell defined as the potential of a cell measured under standard conditionsthat is with all species in their. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it or use the electrical energy supplied to it to facilitate chemical. See also cell potential Standard conditions of temperature and.
Standard conditions are those that take place at 29815 Kelvin temperature 1 atmosphere pressure and have a Molarity of 10 M for both the anode and cathode solutions. Identify the conditions for a standard electrochemical cell. Solution concentrations of 1 M Solute masses of 1 g Temperature of 298 K Pressure of 1 atm.
Pressure of 1 atm b. Solute masses Chemistry 12032022 1710 miathegeek97 Identify the conditions for a. Consider the diagram of the electrochemical cell.
SElECt one or more. Select one or more. Select one or more.
Add the potentials of the half-cells to get the overall standard cell potential. D Solute masses of l. An electrochemical cell based on redox systems 4 and 5.
This standard 100 mL cell features five ground glass ports 1420. In your answer you should include details of the apparatus solutions and the standard conditions required to measure this. Pressure 1013 1013 kPa kPa 1 1 atm atm temperature 298 298 K K 25 25 concentration 1 1 moldm3 moldm 3.
Identify the conditions for a standard electrochemical cell. These ports can be used to mount working reference and counter electrodes as well as other accessories. A solution that cannot dissolve any more solute under the given conditions.
Select one or more. Conditions for a standard electrochemical cell are -- Pressure of 1 atm Solution concentrations of 1 mathrmM Temperature of 298 mathrmK Explanation-An electrochemical cell is. It is therefore important that standard conditions be used.
19 2 Describing Electrochemical Cells Chemistry Libretexts

Principles Of Electrochemical Cells Gaskatel
Solved Review Question 12 Status Not Yet Answered Points Chegg Com

What S Happening With Nanofoods Nanotechnology Food Technology Food Packaging
Comments
Post a Comment